Filter your search
More filters
13 Jobs for Clinical Research - Medical & Scientific
Staffing Company
R&D Manager
Posted by: KeystreamYour new company An international molecular diagnostics organisation operating across the US and Europe, supplying to healthcare systems and corporate partners. Experiencing rapid growth and seeking the right people to take their business to the next milestone, whilst retaining their incredible culture and family-feel environment. Your new role The R&D Manager will be responsible You will manage for managing and delivering a range of R&D projects from the development for the point-of-care diagnostic platform to adapting their current systems. You will also be responsible for managing and coaching a team of R&D scientists and engineers in a fast-paced and pro-active environment. Responsibilities include: Lead in the technical direction of the development of new assays in line with company milestones. Manage the R&D team to deliver project plans for new and existing products aligned with company objectives. Ensure that new products are developed within regulatory guidelines. Manage the smooth running of the laboratory facilities, ensure they are operating within QMS and regulatory compliance. Expand the portfolio of equipment to improve the speed and quality of service that patients receive with their diagnosis. What you need to succeed Educated to higher degree level, ideally possess a PhD in a relevant technical discipline. Experience in managing teams of 5-10 people in a busy environment, and proven experience as a strong, successful leader. Relevant R&D experience in IVD product development, molecular diagnostics or related medical devices. Experience in project management of multi-disciplinary projects. Knowledge and understanding of relevant regulatory and quality compliance procedures. Strong molecular biology or clinical microbiology experience. Strong analytical data analysis skills, including statistics and data interpretation.
Staffing Company
R&D Director
Posted by: KeystreamYour new company An international molecular diagnostics organisation operating across the US and Europe, supplying to healthcare systems and corporate partners. Experiencing rapid growth and seeking the right people to take their business to the next milestone, whilst retaining their incredible culture and family-feel environment. Your new role Manage internal research and development activities in line with company objectives. Provide technical leadership in new product portfolios and technology development. Effective management of financial, human & other resources, necessary to fulfil the responsibilities of the role. Ensure all development projects are compliant to quality and regulatory standards. Drive end to end product development cycles of complex medical devices. Develop and implement R&D tools, processes, standards and metrics, including establishment of best practices. Project and maintain, at a high-profile, the company's scientific excellence to an external audience. Identify and manage external funding and scientific initiatives. Develop strategies for developing & protecting the company's intellectual property. What you need to be successful Educated to higher degree level, ideally PhD, or obtain a Masters Previous experience in a senior research & development role, having led successful teams. Proven track record focused on IVD product development on molecular products, including successful new product launches. Strong working knowledge of Quality Management System & international regulatory requirements. Ability to consistently make timely decisions even in the face of complexity, balancing systematic analysis with decisiveness. Strong strategic leader that effectively leads and guides from the front. Demonstrates quantitative and qualitative analytical and problem-solving skills.
Staffing Company
Medical Scientist
Posted by: Optimum Patient CareYou will have the scope and capability to contribute to the development of our organisation’s medical quality improvement programs. The purpose of the role is to facilitate the translation of research data and quality improvement programs into clinical practice and improvement of patient care. The duties include assisting with drafting and reviewing documentation and publications, project coordination and liaison with project teams, as well as presentation to external stakeholders and conference participation. This is a fantastic opportunity to become involved in medical management and quality improvement projects with an internationally recognised research organisation involved in the analysis and dissemination of real-life research from large-scale observational studies and pragmatic randomised controlled trials. This role can be based in our Cambridge or Norfolk offices on a full or part time basis. For highly experienced individuals remote working may be considered. Send your application to: [email protected] Primary responsibilities Contribute to medical quality improvement programs Continually assess the benefit of our quality improvement programs to patients, and tailor the programs to maximise their real-life application in a clinical setting Review and contribute to scientific documents and publications (including proposals and reports) Independently develop ethics applications for projects Contribute to new and existing projects with respect to considering practical medical issues and clinical application; and to understand EMR data and coding systems and give guidance on the application of data to projects Review and complete presentations, abstracts, and posters Communication and liaison with internal team across various global locations Liaison with study sponsors and steering committee experts Presentations and calls with external stakeholders Participation and presentations at international medical / scientific conferences
Direct Employer
Medical Lab Scientist
Posted by: Healing Stripes HospitalConduct Research and Analysis Calibrate and maintain laboratory equipment to ensure it is functioning at peak levels Write reports summarizing various laboratory findings Maintain laboratory logs to track samples and specimens, recording when these materials are moved to various areas within the lab so they can be accessed quickly as needed Maintain lab safety at all times by following safe handling and quality control processes and wearing proper protective gear Collaborate with other lab staff members and may interact with nurses and other medical professionals to receive various specimens and samples
Staffing Company
VIETNAM. MEDICAL LABORATORY JOBS
Posted by: IMS RecruitmentMEDICAL SCIENTIST / LABORATORY TECHNICIANSSeveral vacancies available for experienced medical scientists / bioscience technicians

Writing Effective Healthcare Job Cover Letters Do Matter

How Important is Body Language in a Medical Job Interview?

How the US Healthcare Job Market Will Change Post COVID-19
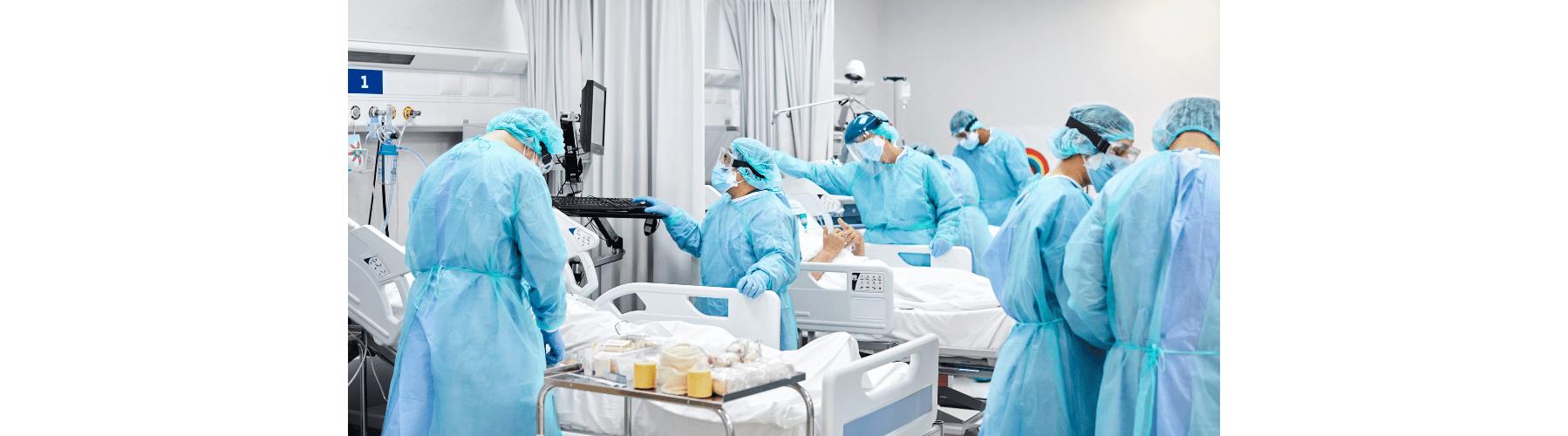
The Challenges and Rewards of Being an ICU Manager: A Journey of Dedication and Impact
Staffing Company
UNIT HEAD - QUALITY CONTROL
Posted by: Big Pharma JobsSupervises the Quality Control functions/activities of a project or its parts, including Quality Control inspection schedules and the collection of Quality Records.Supports the Construction Manager in dealing with the Client for all the Quality matters.Assesses the implementation of the Quality Plan and Quality Control Plans on the site.Allocates Quality Control personnel to the various areas of site activity.Supervises inspections, reports and the documentation issued by inspectors and collect and file the required Quality Records.Evaluates the qualifications of welders and Non Destructive Test-NDT technicians with regard to the activities assigned.Evaluates the qualifications of inspection personnel.Supports the construction roles in the management and control of subcontractors.Supports and participate to all the internal/external audits.Coordinates the relevant Tracking Systems for correct identification of materials.Supervises the correct equipment calibration management activities.Supports the Welding & Non Destructive Test-NDT qualification activities.Cooperates to issue the Welding Procedures (WP).Cooperate with the Project Quality Engineer (PQE) to analyses of non conformities.Supervises inspection of defect renewal and welding.
Direct Employer
The Clinical Lab Scientist, according to departmental policies and professional standards, performs and reviews various moderate to highly complex test procedures in one or more sections of the laboratory without direct supervision. Testing must be requested by a licensed physician and requires a degree of skill that is commensurate with the individual’s education, training, experience and technical abilities.
Staffing Company
Research Medical Officer
Posted by: SD RecruitmentSD Recruitment is looking for a Research Medical Officer for a 2 year contract position to start Monday 23rd August in Crossroads
Staffing Company
CLINICAL LAB SCIENTIST
Posted by: PassionHRWe are looking for a professional, experienced Clinical Lab Scientist in Sitka, AK that is able to work quickly and efficiently while providing excellent patient care. Provider will also maintain a safe and clean work environment by complying with procedures, rules and regulations





